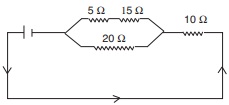
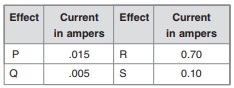
Following are important tips to write 10th Class Public Examinations in English Paper. The tips are useful for the students appearing for 10th Class exams in Andhra Pradesh and Telangana States. You are to be very careful about the instructions given to each question. The answers you write have to be tallied with those in the key of the examiner. Otherwise you may not get maximum marks! So, first of all read the question paper concentrating on the marks allotted for each question.
Also Read: How to Score 10 CGPA in 10th Class Exams
Commit To Memory:
Don’t forget that you have to write all the answers in the answer booklet supplied to you along with the question paper.
Do’s:
For multiple choice answers, write the numbers and letters clearly, as 1. A, 2. B….
Don’ts:
In no case, you don’t overwrite the numbers or rewrite congestedly. Instead, you can round off the wrong ones and rewrite clearly taking a clear gap in between so that your answer is clearly visible.
Do follow it for the entire answers in the answer booklet. Don’t forget that you can get maximum marks for the neatness of the answer booklet. So try to please the examiner by your neat writing and correct answers. Maintain the required gap between words in each sentence and use punctuation marks where required and follow the division of paragraph for essay type answers.
Also Read: 10th English – Tips on Answering Creative Writing Questions
Reading Comprehension:
Under Reading Comprehension, passages from the prescribed text book as well as from other source are asked in the examination.
Students have to answer a number of questions. There are three important steps to attempt a comprehension viz, reading, understanding and answering the questions set on the given passage.
Comprehension is a test of understanding and analysing a passage. Practice of comprehension passages will boost the student’s vocabulary. You can write correct answers only when you fully grasp the meaning of the passage.
Since the aim is to read the passage thoroughly and seek out the meaning, it may not be apparent upon the first reading.
Do’s:
So, you have to express the meaning of the passage in your own simple, clear and direct English. Do remember that you have a clear understanding of the questions asked!
Read the passage(s) carefully two or three times, till you understand clearly – the subject and what is said about the subject, that is the theme of the passage.
Read carefully the questions one by one and find out if you thoroughly understand them to the level of writing answers to them. Now take up the questions one by one and find out the parts of the passage they refer to.
Revise your answers and examine them to see that they are clear and complete. Correct if there are spelling mistakes, grammatical errors or lapses in using punctuation marks.
Don’ts:
You should never copy the language of the passage. That is, the sentences should not be full of ornamentation or complex. Instead, your answers should be in your own words – simple and direct. They should not be too short nor too long but should be in accordance with the instructions given under the the passage(s).
Examiners cannot award you marks if they are unable to understand what you are trying to say. Beware of just copying from the passage without really understanding what you are writing.
Since passages under Reading Comprehension are given from both the prescribed text book and from other source a thorough reading of the lessons under Reading – A, Reading – B and Reading – C of the text book is a must. Extensive and Intensive Reading of the lessons is desirable. Also make it a practice reading other various
books, periodicals and preferably newspapers. Text books of other classes, VI, VII, VIII and IX have to be studied again and again.