Question: Write down the characteristics of the elements having atomic number 17. (4 Marks)
a) Electronic configuration …. b) Period number …..
c) Group number ….. d) Element family …..
e) No. of valence electrons ….. f) Valency …..
g) Metal or non-metal …..
Answer: a) Electronic configuration: 1s2 2s2 2p6 3s2 3p5 (Z = 17)
b) Period number: 3rd period
c) Group number: VII A group (17th)
d) Element family: Halogen
e) No. of valence electrons: 7 (2 + 5)
f) Valency: 1 (8 – 7)
g) Metal or non-metal: Non-metal
———————————
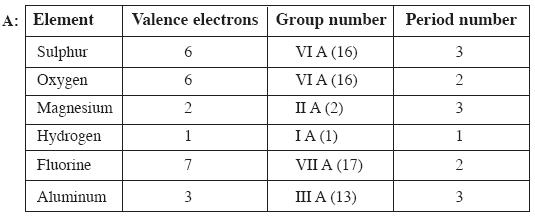
Question: a) State the number of valence electrons, the group number and the period number of each element given in the following table. (AS – 1) (4 Marks)