Question: In the period 2, element X is to the right of element Y. Then find which of the elements have
I) Low nuclear charge II) Low atomic size
III) High ionization energy IV) High electronegativity
V) More metallic character (AS – 1) (4 Marks)
Answer:
I) Low nuclear charge: Element Y has low nuclear charge.
II) Low atomic size: Element Y has low atomic size.
III) High ionization energy: Element Y has high ionization energy.
IV) High electronegativity: Element Y has high electron negativity.
V) Metallic character: X has more metallic character.
———————-
Question:
How does metallic character change when we move
I) Down a group II) Across a period. (AS – 1) (2 Marks)
Answer:
I) Down a group: Metallic character of elements increases as we move down a group.
II) Across a period: Metallic character of elements decreases as we move across a period.
——————–
Question:
Why was the basis of classification of elements changed from the atomic mass to atomic number? (AS – 1) (4 Marks)
Answer: At the time (1829) when Dobereiner established the law of triads electrons were not discovered. With the available information of atomic weights he established the law of triads.
Even at the time of John Newlands (1865) with the same available data of atomic weights of elements he established the law of octaves. The same process continued with Mendeleev while developing the periodic table.
Mendeleev’s periodic table faced limitations and the anomalous pair of elements, and dissimilar elements placed together paved way to put the minds of the scientists on this issue.
Incidentally in 1913 Moseley’s X-ray analysis introduced the concept that the atomic number is more fundamental characteristic of an element than its atomic weight. As a result the modern periodic law is stated as ‘The properties of the elements are periodic function of their atomic number’.
———————–
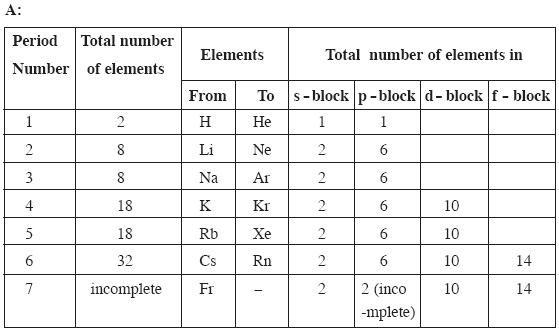
Question: Complete the following table using the periodic table. (4 Marks)